We are introduced to PV diagram, which makes it easier to find the work done by gas.
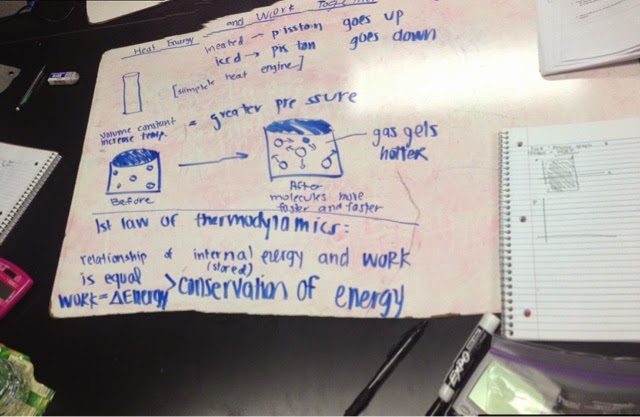
By making a simple heat engine, we are going to make some assumptions. What will happen when the volume is constant but the temperature is increasing? We assume heat will make pressure goes up, and ice water will make it go down. And the pressure will be greater as gas gets hotter since molecules move faster and faster.
We get to the first law of thermodynamics. which is the relation of internal energy,heat and work. deltaU(internal)=Q-W. It is also called the conservation of energy.
What if W =0? In real life, we are given some examples, such as in souna and heating water.
What if Q=0? In real life, ball rolling down hill and spring are both good examples.
Then, we do an exercise for kinetic energy using kinetic molecule model. Through a long process, we deduct the conclusion that:
Root-mean-square speed of a gas molecule formula is v=(3RT/M)^0.5
Root-mean-square speed of a gas molecule formula is v=(3RT/M)^0.5
Average transnational energy of n moles of ideal gas =3/2 nRT
Average transnational energy of a gas molecule=3/2 kT
ave transnational energy per mole of gas =3/2 Rt
The graph shows us the most probable speed of molecules are on the left of avg speed and rms.
Through the following prove process, we get the relationship of T V in adiabatic process
This is a problem applying the adiabatic formula
Finally, we do a problem about fire syringe. Since it is most diatomic gas, we should use 5/2 instead of 3/2.
Here is the video of fire syringe













No comments:
Post a Comment